Darkstone42
Oh.
What's with the avatar of you making coffee DS?
Your old one got my vote for the Hooplas.
Why thank you for your vote. Methyl Red will likely make its return once I start my senior project. Or perhaps I'll go with Disperse Orange 3. Or maybe one of the long-chain elastomers I synthesize will be the go-to compound. I guess we shall see.
The new picture is indeed of pipetting. I believe I was adding a pH 7 monobasic phosphate buffer to my solution. I was studying horseradish peroxidase inhibitor kinetics in the spirit of cancer treatment research. That was probably one of the trials in which I was testing the inhibition potential of ibuprofen.
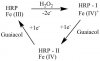
For those of you to whom it means something, I was observing steady-state kinetics of the reaction I attached, which is catalyzed by the HRP enzyme. In the pro-drug treatment being proposed, the guaiacol would be replaced with acetaminophine, but the two act analogously and guaiacol is cheaper. We were testing acetylsalicylic acid and ibuprofen since other phenol-based compounds were found to inhibit the reaction, and these, of course, being found in over-the-counter medications needed to be tested to see if they would make the cancer treatment less effective. Acetylsalicylic acid was determined as an inhibitor, but ibuprofen did not appear to be, for what it's worth.
The device we were using to observe the kinetics was an Ocean Optics spectrophotometer, using Spectra Suite software, and the reaction took place in a quartz cuvette with a 1-cm optical pathway. We used 22.9 nM HRP, 1.08 mM hydrogen peroxide, ibuprofen concentrations varying from 1 mM to 6 mM, and guaiacol concentrations ranging from 10 uM to 3 mM.